Draw all b Draw the structure of ethane in skeleton mode or as a hydrogen atoms bond-line structure. Draw all hydrogen atoms.
Solved Ethane Is An Organic Compound With The Molecular Chegg Com
Include all hydrogen atoms Expert Answer.

. The complete ethane chemical formula and its structural formula are given in the following points. A Draw the complete structure of ethane. So thats the structure of meeting similarly the word that literally means to carbon atoms and since there is in here it would also imply that there will be a single one between carbon atoms.
A hydrogen atom has 1 electron in its outer shell. Draw the complete structure of ethane. The suffix ane indicates that compound has no unsaturation so double bond is not present and all the four valency of carbon are.
Let us draw a Lewis structure of ethane step by step. A draw the complete structure of ethane. Eyes as we know one carbon atom can form four bombs.
On each carbon atom there are 3 hydrogen atoms. It has a chemical formula of C 2 H 6 and is a hydrocarbon. Draw all hydrogen atoms b Draw the structure of ethane in skeleton mode or as a bondline structure.
Do not draw the hydrogen atoms Select Draw Rings More Erase Select Draw Rings More Erase. CH_3-CH_3 All the bonds present in the ethane molecule are covalent bonds. A carbon atom has 4 electrons in its outer shell.
Since the general formula for ethane is CₓH₂ₓ₂ the condensed formula is C₂H₆. What is the Structure of an Ethane Molecule. A Draw the complete structure of ethane.
See the answer See the answer done loading. This molecule formula is CH four. The Electron dot structure for ethane molecule is.
Ethane exists in nature as a flammable colorless and odorless gas. The chemical or molecular formula of ethane is-. A bond is formed when two atoms have a mutual sharing of electrons.
Ethane Chemical Formula Ethane has 2 carbon atoms with 6 hydrogen atoms attached to them. Ethane has quite many uses in various industries and has one of the most simple. Determining the total number of valence electrons in the molecule.
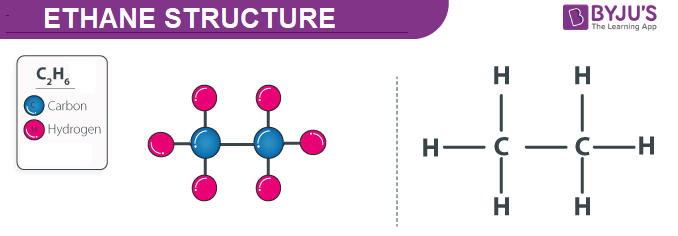
The Ethane structure is discussed below. It is a two-carbon containing saturated hydrocarbons consisting of only sigma bonds. The chemical formula of Ethane is rmC_2rmH_6 and its chemical structure is shown below.
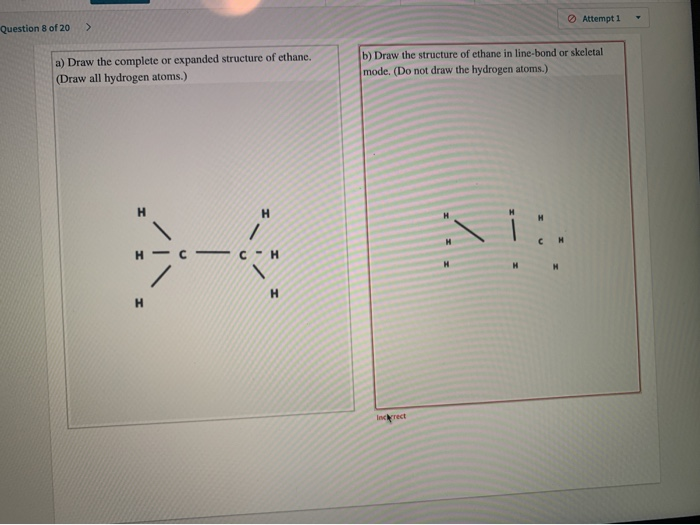
ADraw the complete structure of ethane. I ethane ii ethene and iii ethyne. Do not draw the hydrogen atoms.
It is a colorless and odorless molecule that exists as a gas at the standard room temperature. The structural formula of ethyne. Ethyne also known as acetylene is an organic chemical compound with the chemical formula C 2 H 2.
H C C H. Carbon has 4 valence electrons represented as x in the diagram and each hydrogen atom has 1 electron represented a in the diagram. Science for Tenth Class - Part II - Chemistry.
8 rows Ethane is a gas and therefore volatilization from soil and water is expected to be the most. Ethane is an organic compound with the molecular formula C H. Write the electron dot structures for.
Include all hydrogen atoms. Include all hydrogen atoms. Because the chemical structure only consists of single bonds between.
The ethane formula is eqC_2H_6 eq and the chemical structure is shown in the diagram above. B Draw the complete structure of propane. Complete Step by Step Solution.
Up to 256 cash back Get the detailed answer. Ethane is an alkane which consist of two carbon atoms. The chemical structure for the ethane molecule is given below.
Ethyne is regarded by many to be the simplest alkyne since it consists of only two carbon atoms which are triply bonded to each other. Hydrocarbons can have single double or. Carbon atoms can participate in up to four chemical bonds.
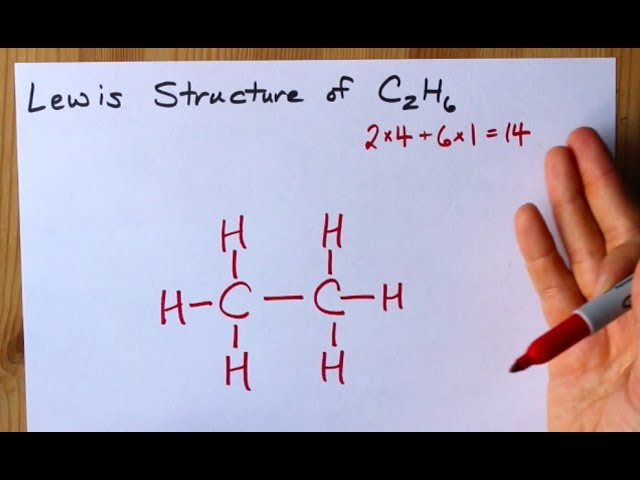
Carbon is in group 4 of the periodic table. In ethane we have two carbon atoms and 6 hydrogen atoms and hence the total number of valence electron are 2 X 4 1 X 6 14. Ethane is only composed of carbon and hydrogen atoms so it is classified as a hydrocarbon.
Ethane is an organic compound with a chemical formula of C2H6. This problem has been solved. The six hydrogen atoms will each share their 1 electron with carbon to form six carbon - hydrogen covalent bonds.
Who are the experts. This compound is one of the simplest hydrocarbons to exist having a single bond between carbon atoms. Ethane has the chemical formula of C 2 H 6 which means that it has two carbon atoms and six hydrogen atoms.
The structural formula or the line-bond structure is shown in the attached picture. So well draw all four ones three with hydrogen normally carbon. NCERT Solutions For Class 12.
Ethane is an organic compound with the molecular formula C2H6C2H6. It has a molecular weight of 3007 gmol and is the second simplest organic compound after methane. Pure ethyne is known to be highly unstable.
Now let us answer the question. The valence electron for carbon 1s22s22p2 and hydrogen 1s1 is 4 and 1 respectively. Up to 256 cash back Get the detailed answer.
We can draw the electron dot structure of ethane as- Formula of ethane is C 2 H 6 In ethane there are two carbon atoms which are singly bonded to each other. Hydrogen can only form 1 bond.

Two Dimensional Molecular Structure Of Ethoxyethane Molecular Molecular Structure Chemistry

From Gen Chem To Org Chem Pt 7 Lewis Structures Master Organic Chemistry Organic Chemistry Molecular Geometry Organic Chemistry Books
Ethane Structure Properties And Uses Of C2h6

Skeletal Formulae Of Organic Molecules Are A Useful Concise Way To Describe The Molecular Structure Of O Organic Molecules Skeletal Formula Molecular Structure

Edexcel Igcse Chemistry Triple 1 44 Draw Diagrams Representing The Positions Delicate Jewelry Necklace Wedding Jewelry Photography Beautiful Diamond Earrings
Lewis Structure Of C2h6 Ethane Youtube
Solved Attempt 1 Question 8 Of 20 A Draw The Complete Or Chegg Com

Ethane Structure Uses Formula Video Lesson Transcript Study Com
0 comments
Post a Comment